At this writing, most of us are in the midst of stay at-home orders due to the SARS-CoV-2 virus pandemic. The world at large is learning more than they wanted to about what viruses are and how difficult they are to control. Plant viruses are not all that different from human viruses. Plant viruses only infect plants and, like human viruses, just as difficult to manage. This document will cover what plant viruses are, how they are transmitted, symptoms they induce in plants, detection methods, and virus management tactics.
Dead or Alive?
I remember the look on a Master Gardener’s face when I told class that most plant viruses do not have DNA, the genetic material found in nearly all living organisms. The look was a cross between puzzlement and deep thinking. She asked, “So they are not alive?” In essence, that is right, viruses are not alive as we generally think about the word.
Plant viruses cannot replicate themselves outside of the plant and have no metabolic activity of their own. They need the host plant to make more copies of themselves. Virus particles don’t have organs or organelles like bacteria or fungi. A plant virus typically consists of a protein outer coat (capsid) surrounding a single strand of RNA. These viruses might look like a rod- or sphere-shaped particle in an electron microscope. The RNA of the virus codes for the plant to make more virus particles. It might be better to think of viruses as infectious molecules.
There are many variations on this simple view of a plant virus. Lettuce necrotic yellows virus and Tomato spotted wilt virus have an outer lipid membrane called an envelope that helps them bind to plant cells (Lucas 2010). Some plant viruses are comprised of DNA rather than RNA where the DNA may be double-stranded (such as Cauliflower mosaic virus) or single stranded (such as Maize streak virus). Many plant viruses, including Beet curly top virus and Cucumber mosaic virus are comprised of two or more particles. There are other plant viruses that are more complex in form and chemical composition.
The official definition of a virus is – “Replicators that encode structural proteins that encase their own genome.”
Transmission
Plants don’t cough or sneeze, disseminating viruses like animals/humans do and viruses don’t have legs or wings to move around. Plant viruses must hitch a ride from an infected plant to a healthy plant. They do this through a great variety of mechanisms including the use of pollen, seeds, natural root grafts, insects and mites, nematodes, fungi and fungal-like organisms and/or man.
Humans are the best vector of plant viruses, especially when propagating new plants. Sourcing new plants from virus-infected mother stock plants is an efficient way of producing many virus-infected plants. Asexual propagation of plants via cuttings or grafting can easily move viruses. Contaminated equipment used in propagation or harvest, such as cutting knives, can move viruses that are mechanically transmitted. For example, Asparagus virus 2 is transmitted mainly through infected sap on cutting knives during harvest and contributes to reductions in asparagus vigor and productivity (Shimura et al 2013).
Virus infected pollen or seed is another way viruses can move to new plants. Prunus narcotic ringspot virus (PNRSV) and Prune dwarf virus (PDV) are common in stone fruit orchards since they can be disseminated to healthy trees through infected pollen (Pallas et al 2012). Bees can also be an unwitting vector moving virus-infected pollen as with Blueberry shock virus (Pscheidt and Harper 2014). Seed transmission can occur when viruses in mother plant tissues spread into seed parts and from the seed embryo into seedlings (Mink 1993). Although viruses can replicate in pollen or contaminate pollen surfaces they may not be naturally transmitted to healthy plants.
Insects and eriophyid mites are vectors for the majority of plant viruses (Hohn 2007; Whitfield et al. 2015). Aphids, leafhoppers, thrips, whiteflies, and some beetles can be virus vectors. Aphids feed on plants using a stylet that pierces plant cells. Viruses may be simply picked up in and/or on the stylet when an aphid feeds on a virus-infected plant. The virus can then be moved when the aphid feeds on a healthy plant. The ability of an aphid to transmit the virus may only last for a few minutes to hours. This quick type of transmission is called non-persistent or non-circulative, stylet-borne transmission (Cauliflower mosaic virus for example). If the ability to transmit the virus lasts a few days and involves the virus being contained in the foregut of the insect (as with Lettuce infectious yellows virus and its whitefly vector), this is called non-circulative, semi-persistent transmission. There are some plant viruses, such as Barley yellow dwarf virus, that in essence infect the insect where the virus circulates through the body to end up in the salivary glands of the insect. The insect can then transfer the virus to any healthy plant when feeding for the rest of its life; this is called persistent, circulative transmission. Sometimes the virus can also be inherited by the offspring of these insects.
In some cases, the virus may alter the behavior of the insect. Insect vectors may prefer healthy plants when the insect has been infected by the virus or the vector may prefer infected plants when the insect does not contain the virus. This behavioral change helps transmit the virus from infected to healthy plants. (He et al 2015, McMenemy et al 2012)
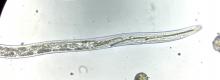
Dagger nematodes in the genus Xiphinema are capable of transmitting plant viruses as well (Pinkerton et al 2008). These
soil living plant parasitic nematodes have a stylet mouth part. Nematodes feeding on the roots of an infected plant acquire the virus and subsequent, feeding on the roots of a healthy plant, transmit the virus. Tomato ringspot virus can be transmitted between plantings of tree crops through a combination of infectious nematode survival between crops and/or other infected host plants (including weeds) that also grow and survive between crops (Hadidi et al 2011).
Fungi and fungal-like organism can also transmit plant viruses. The oomycete Olpidium virulentus transmits Mirafiori lettuce big-vein virus, the causal agent of lettuce big vein disease (Lot et al 2002). The soilborne fungus-like organism Polymyxa graminis transmits Wheat soilborne mosaic virus (Rao and Brakke 1969).
Symptoms and Diagnosis
Virus infection of plants can result in a wide variety of symptoms including symptoms usually associated with other pathogens or abiotic factors, very specific symptoms that are only ever associated with virus infection and nondescript symptoms related to ambiguous problems. Viral infections may also not produce any symptoms at all. Combinations of symptoms can be found in many virus-infected plants. Symptom development may be dependent on environment such as symptoms developing under cool conditions but not occurring in newer tissue when the temperature is warmer. Diagnosis can occasionally occur through symptoms alone,
however, it must be confirmed by detection of the virus itself.
The following are common symptoms associated with virus infection:
Color breaks – Flower petals develop stripes, streaks, or feathering of different colors. The color variation is related to the local accumulation of pigments in the upper epidermal layer. The lighter colors appear as irregular streaks or fine feathering. Dark breaking occurs when the color in the epidermal cells intensifies in small dark streaks or elongated flecks. These can be easily confused with genetic variegation.
Enations – An abnormal outgrowth from the surface of a stem or leaf. Generally found on the underside of a leaf, these can take the shape of leafy outgrowths or just raised protuberances between lateral veins and along the midrib. Can also occur on fruiting structures such as pea pods. This symptom is unique to virus infection.
Leaf and flower flecks – A small, white to translucent lesion (spot) that is visible through a leaf or flower. Flecks may also be necrotic.
Line patterns – Chlorotic zigzag or wavy lines that develop in leaves. These are sometimes called an oak leaf pattern when the colored lines look like the outline of an oak leaf within the virus-infected leaf.
Mosaic – A non-uniform foliage coloration where patches of normal green, light green, dark green and/or yellowish patches are intermingled. Mosaics can also occur in fruit.
Mottle – An irregular pattern and merging of light and dark areas that develop in leaves but are not in patches as occurs with a mosaic.
Phyllody – Change from a normal flower to leafy structures. Also common for certain phytoplasma infections.
Ringspot – Yellowish or dead (necrotic) rings with green tissue inside them found on leaves or flowers.
Rosetting – A short, bunchy growth habit of shoots due to shortened internodes along with no comparable reduction in leaf size.
Rugose – The affected structure has a wrinkled or roughened appearance.
Shothole – The dropping out of small, round fragments of leaves, making the leaf look as if it was riddled by gun shot.
Stem Pitting and Black Line – Death of the vascular cambium that occurs in small sections along the trunk of woody perennials and results in depressions and/or groves in the xylem (wood) that can be seen when the bark is stripped away. When a susceptible plant is grafted to a resistant plant, death of cells immediately between the two can occur (a hypersensitive response) when the susceptible plant is infected with the virus resulting in a black line between the two genotypes.
Streaking – an elongated lesion with irregular sides.
Stunting or dwarfing – A poorly growing plant that is reduced in size and vigor.
Variegation – A mosaic with bright yellow angular patches on green leaves.
Veinal associations including veinal chlorosis (yellowing or reddening), vein clearing (loss of color), or veinal necrosis (veins turn brown). Similar color changes can occur between the leaf veins (interveinal chlorosis or necrosis) but interveinal symptoms may be due to nutritional deficiencies rather than a virus infection.
Virus infections can also be associated with leaf curling and other leaf distortions, thickening of leaves, necrotic spots on leaves and flowers, and dieback of shoots.
Unfortunately the naming of viruses often started by including the name of the first host it was found on along with typical symptoms. This resulted in some viruses with wide host ranges being called many different names. For example, Cucumber mosaic virus (CMV) was first found in cucumbers but infects many other plants and was also known as tomato fern leaf virus, pea western ringspot virus, lily ringspot virus, peanut yellow mosaic virus and soybean stunt virus just to name a few. Further complicating the issue was finding that two or more viruses coinfecting the same plant may be needed to produce the symptoms generally associated with one viral disease (Quito-Avila et al 2014). Mixed viral infections are so frequent that they should be considered the rule more than the exception (Moreno and Lopez-Moya 2020).
Although some symptoms are only associated with viruses, an accurate diagnosis can only be verified by detecting the virus itself. Modern virus detection involves serological testing (use of antibodies) or molecular methods. Test kits based on these methods are available to detect certain viral infections in plants while in the field. In most cases, however, detection of plant viruses will need to be determined by a public or private laboratory.
If present and stained correctly, some virus infections can be identified when aggregates of viral particle known as inclusion bodies are viewed with a light microscope (Christie and Edwardson 1994). These aggregates of viral particles can produce interesting forms that look like pinwheels, crystals, hexagonal, stacked, or rounded plates. Their composition, shape and tissue location can help identify specific virus or viral groups.
Management Tactics
There are no chemical treatments available that eliminate viruses once plants are infected. Only cultural tactics can be effectively used to prevent the introduction of viruses into a healthy field. The number one tactic is to use planting stock that has been certified as virus-free, plant material that has been tested and shown to be free of all known viruses that affect the crop (Hadidi et al 2011). Certified virus-free plant material is superior to just “tested” or tested for only a few viruses.
Propagating plants from symptomless but virus-infected plants has devastated many growers of perennial crops. Virus symptoms may require more than one growing season to develop or may occur under specific environmental circumstances, when another virus is introduced, or when grafting onto new rootstocks or scions. Symptomless but virus-infected plants may be brought into a new production area from other areas of the country or world.
Select plant cultivars or varieties with resistant or tolerant to common viruses, if available, to avoid a repeat of damage caused by a given virus.
Planting location can also be an important consideration. Locate new fields upwind or isolated from older virus-infected fields. Plant in large blocks to slow movement of pollenborne viruses into new plantings, especially if fields in the surrounding area are infected.
Vector control can be very useful for some virus diseases. Test soil in spring for dagger nematodes. If dagger nematodes are found, then locate the field in a different place or fumigate the soil to reduce the nematode populations. Management of insect vectors is helpful to prevent infection and reduce spread. This is more effective for those viruses that are spread in a persistent manor. Viruses spread in a non-persistent way may be transmitted too quickly before control tactics for the vector can take effect.
Removal of infected plants (rogueing) is critical when trying to prevent spread within a field or when propagating. This may only be effective when symptoms occur on the plant in a time frame before spread can occur. Rogueing may not be effective if there is long latent period between infection and symptom development.
Heat-treatment of scion stock plants before grafting can be effective in some cases. Temperatures need to be carefully or precisely maintained to kill the virus but not the plant. Tissue culture propagation of shoot tips can also be effective if the virus does not initially occur in the meristematic area of the plant.
When propagating plants susceptible to mechanically-transmitted viruses, disinfect cutting knives and other tools frequently by soaking 60 sec in 10% bleach, or shellac thinner (70% ethyl alcohol), or quaternary ammonium. Surfaces contaminated with Tobacco mosaic virus can be disinfested using a 20% solution of nonfat dry milk (plus a surfactant) (Lewandowski et al 2010).
In some specific situations it might be advisable to do nothing but let the disease run its course. Blueberry shock is pollen-borne but plants recover from infections. It is not cost effective to rogue blueberry plants with this virus from large plantings. Plants that have recovered from the symptoms appear to produce a full crop, but these plants can continue to serve as an inoculum source for nearby plants and any new plantings (Pscheidt and Harper 2014).
References
Christie, R. G., and Edwardson, J. R. 1994. Light and Electron Microscopy of Plant Virus Inclusions. Florida Agricultural Experiment Station Monograph Series, No. 9, rev.
Hadidi, A., Barba, M., Candresse, T., and Jelkmann, W. 2011. Virus and Virus-like Diseases of Pome and Stone Fruits. St. Paul, MN: APS Press.
He, W. B., Li, J., and Liu, S. S., 2015. Differential profiles of direct and indirect modification of vector feeding behavior by a plant virus. Scientific reports, 5 article number 7682.
Hohn, T. 2007. Plant virus transmission from the insect point of view. Proceedings of the National Academy of Sciences, 104:17905-17906.
Lewandowski, D. J., Hayes, A. J., and Adkins, S. 2010. Surprising results from a search for effective disinfectants for Tobacco mosaic virus-contaminated tools. Plant Disease 94:542-550.
Lot, H., Campbell, R. N., Souche, S., Milne, R. G., and Roggero, P. 2002. Transmission by Olpidium brassicae of Mirafiori lettuce virus and Lettuce big-vein virus, and Their Roles in Lettuce Big-vein Etiology. Phytopathology, 92:288-293.
Lucas, W. 2010. Viral Capsids and Envelopes: Structure and Function. Wiley Online Library DOI: 10.1002/9780470015902.a0001091.pub2
McMenemy, L. S., Hartley, S. E., MacFarlane, S. A., Karley, A. J., Shepherd, T. and Johnson, S. N. 2012. Raspberry viruses manipulate the behavior of their insect vectors. Entomologia Experimentalis et Applicata, 144:56-68.
Mink, G. I. 1993. Pollen and seed-transmitted viruses and viroids. Annual review of phytopathology, 31:375-402.
Moreno, A. B. and López-Moya, J. J. 2020. When viruses play team sports: Mixed infections in plants. Phytopathology, 110:29-48.
Pallas, V., Aparicio, F., Herranz, M. C., Amari, K., Sanchez-Pina, M. A., Myrta, A., and Sanchez-Navarro, J. A. 2012. Ilarviruses of Prunus spp.: A continued concern for fruit trees. Phytopathology 102:1108-1120.
Pinkerton, J. N., Kraus, J., Martin, R. R., and Schreiner, R.P. 2008. Epidemiology of Xiphinema americanum and Tomato ringspot virus on red raspberry, Rubus idaeus. Plant Disease 92:364-371.
Pscheidt, J. W., and Harper, N. 2014. Progression of symptoms on blueberry infected with Blueberry shock virus. Plant Health Progress doi:10.1094/PHP-RS-13-0121.
Quito-Avila, D. F., Lightle, D., and Martin, R. R. 2014. Effect of Raspberry bushy dwarf virus, Raspberry leaf mottle virus, and Raspberry latent virus on plant growth and fruit crumbliness in ‘Meeker’ red raspberry. Plant Disease 98:176-183.
Rao, A. S., and Brakke, M.K., 1969. Relation of soil-borne Wheat mosaic virus and its fungal vector, Polymyxa graminis. Phytopathology, 59:581-587.
Shimura, H., Masuta, C., Yoshida, N., Sueda, K., and Suzuki, M. 2013. The 2b protein of Asparagus virus 2 functions as an RNA silencing suppressor against systemic silencing to prove functional synteny with related cucumoviruses. Virology, 442:180-188.
Whitfield, A. E., Falk, B. W., and Rotenberg, D. 2015. Insect vector-mediated transmission of plant viruses. Virology 479-480: 278–289. http://dx.doi.org/10.1016/j.virol.2015.03.026