Spotted-wing drosophila (SWD; Drosophila suzukii; Family: Drosophilidae) is a key pest that targets a wide variety of susceptible fruits including tree stone fruits (e.g., cherry) and berries (e.g., strawberry, blueberry, raspberry, blackberry, and wine grape). SWD is widespread throughout all the important production regions in the U.S., Europe, and South America and originates from Asia.
Pest description, fruit damage, and life cycle Adult SWD flies resemble common vinegar (pomace) flies and sometimes are referred to as fruit flies. SWD can damage ripe-to-overripe fruit by depositing eggs directly beneath the surface. Larvae develop from these eggs, resulting in unmarketable fruit. Drosophila suzukii can survive on a range of non-crop hosts in surrounding vegetation, which makes control more difficult.
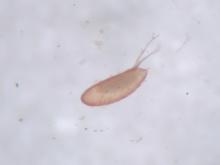
Identification SWD have red eyes and a yellowish-brown amber colored body measuring 0.125 inch (2-3 mm) in length. Two key characteristics distinguish SWD from other common fruit flies: 1) a black spot (sometimes dark, sometimes faded) near the leading top edge of adult male wings (females do not have wing spots); 2) the prominent saw-like ovipositor (used to insert eggs into fruit) on the female’s posterior end; and 3) the pupae of both sexes have two respiratory horns sticking out of the anterior end; each horn with a complete whorl of 6-8 fingers/points. Several dark, continuous bands are visible around the abdomen of both the male and female. A hand lens and good lighting are useful for seeing these characteristics. Note: if the female gets caught in a liquid bait (e.g., apple cider vinegar or yeast) within a monitoring trap, her ovipositor may extrude from the body (because of the liquid), thus aiding in the identification of female SWD. See references below.
Symptoms from damage To recognize SWD damage, look very closely for scarring or spotting on the fruit surface; liquid exuding (when squeezed) out of scar/hole where eggs were laid; softening, collapsing and/or bruising of fruit at damage site; small white larvae and pupae that can be seen with naked eye if fruit is opened; and, under a microscope, two hair-like filaments sticking out of fruit where they are connected to a white egg within the fruit can sometimes be seen. See OSU Extension Bulletin EM 9021, Recognize Fruit Damage from Spotted Wing Drosophila (https://catalog.extension.oregonstate.edu/em9021).
Life cycle A single female can lay several hundred eggs. Adults can live 3 to 4 weeks in summer and several months in winter. Larvae feed inside the fruit for about 5 to 7 days, until they are ready to pupate. Most larvae pupate outside of the fruit. Non-feeding pupae are brownish yellow with star-like (6 to 8 points) respiratory horns at one end. Pupation lasts for 3 to 10 days and emerging adult flies will mate, producing additional offspring. Adult SWD use alternative hosts in surrounding vegetation to survive the winter period. Several generations commonly occur in Oregon, depending on environmental conditions during the cropping season.
Sampling
Like most fruit flies, SWD are attracted to the odors of rotting fruit, i.e., fermentation, vinegar, and alcohols. To construct simple homemade traps, use any plastic container (18- to 32-ounce size) with a removable lid. Drill small holes around the middle of the cup to allow flies to enter. Holes should be small to limit the ability of larger insects to enter (3/16 in or 4.76 mm). Leave an undrilled space on one side of the cup to make it easier to pour out the liquid bait solution when checking the trap and changing the solution. Add a couple of inches of liquid bait such as apple cider vinegar and white wine to the cup. Adding a drop of liquid soap to the solution will break the surface tension of the liquid so that captured flies sink more easily. An alternative bait that catches even more flies can be made from a mixture of yeast, sugar, and water (2 teaspoons of dry baker’s yeast, 4 teaspoons of sugar, and 1.5 cups of warm water). The yeast and sugar solution makes identification of SWD, from all the other flies, more difficult. Commercial traps and lures are available from Scentry Biologicals Inc. and Tréce Inc. and can be easily purchased through Great Lakes IPM (greatlakesipm.com).
Traps should be hung within the canopy of the crop or close to the fruit level when possible. Avoid placing traps in direct sun as midday heat inside the trap can reduce catch. For strawberries, secure the trap on the ground within the plants and construct a roof structure to provide shade (red plastic plates work well). Traps can also be placed in locations where flies are likely to be intercepted while moving through the landscape, for example fence lines near adjacent non-crop plants and borders shared with other fruit growers.
In most commercial settings protective (prophylactic) sprays are applied, regardless of catch data from traps, because damage thresholds are zero. In organic production and backyard fruit, more traps can help with early detection and improve pest management decisions. Check and refresh traps once a week. While mass trapping has not been shown to be an effective control tactic, traps sometimes catch thousands of SWD, so multiple traps can help reduce populations.
Male SWD can sometimes be identified with the unaided eye by looking for the dark spots on the male’s wings. It is difficult to confirm SWD and see the female’s ovipositor without a magnifying glass or microscope. Traps sometimes catch so many insects that it is difficult to make out what was caught. For a closer look, filter the contents of your traps using a strainer, and place insect in a pan with a solid white background. Spread the insects out (using a small paintbrush or tweezers) and examine contents with a magnifying lens or under a microscope. Numerous species of Drosophila and other insects will also be attracted to these traps, especially if the yeast bait is used. Damage thresholds have not yet been established for this pest.
Fruit inspection
Several methods have shown positive results for recovering larvae by using a salt or sugar solution over crushed fruit. Collect ripening fruits suspected to be infested with SWD in a plastic bag. See “A quick, 7-step guide for detecting larvae in fruit” (OSU Extension Bulletin, EM 9097) and “A Detailed Guide for Testing Fruit for the Presence of Spotted Wing Drosophila (SWD) Larvae” (OSU Extension Bulletin, EM 9096).
Preparation of extraction solution: Dissolve 1 cup of plain salt in 1 gal warm water (10 BRIX); –or– 2.5 cups of brown sugar in 1 gal water (16 BRIX). Solutions must be thoroughly dissolved to help larvae float on top of the solution for easy viewing. Prepare the solution in advance, if possible.
Extraction method: Place a layer of crushed fruit in a shallow white pan. Pour solution (salt or sugar) over crushed fruit. A good proportion of larvae will exit the fruit after a few minutes, looking for air at the top of the liquid. A majority will float to the surface, unless stuck in or under pulp. Wait for at least 15 minutes to get a majority of larvae out of infested fruit. Look for moving white larvae on the surface of the liquid. Eventually, however, they will die and sink to the bottom of the pan. The sugar solution will keep them alive longer than salt solution. Do not mistake SWD larvae for plant parts, fruitworm, thrips, aphid skins, other Drosophila larvae, etc.
Fruit dunk bag method: Place suspect fruit in a large sealable plastic bag. Crush fruit by using a rolling pin over bag or squeeze/crush fruit with hands through bag. Add solution to the bag of crushed fruit. Shake bag lightly to promote penetration of solution into the fruit. If fruits are infested, white SWD larvae will float to the top, and fruit should settle on the bottom (note: some fruit floats, depending on fruit weight/amount and sugar levels in fruit). It may take over 15 minutes or so for larvae to float and fruit to separate. If larvae are small, a hand lens or scope and good lighting are useful to see their presence. Hold the clear bag in light and small larvae may be seen moving and floating among fruit.
Management—cultural and physical methods
Harvest in a timely manner—Pick fruit at regular intervals to prevent egg-laying opportunities and SWD infestations. Avoid leaving overripe fruit to hang on host plant.
Clean up infested fruit—To avoid SWD populations from increasing, clean up overripe hanging, fallen, and SWD-infested fruit.
Create a barrier—If a monitoring program detects SWD, cover fruiting clusters (e.g., blueberry) or entire fruiting plants (e.g., caneberry) with a fine netting (less than 1 mm in size if feasible) to reduce egg-laying. Use weed fabric, and control weeds as they provide a more suitable habitat for SWD. Weed fabric reduces the survival of pupating larvae.
Protect fruit from damage—Protect fruit from rain and sun to reduce fruit splitting and to improve fruit quality. SWD can be attracted to damaged fruit.
Select early season fruit cultivars to reduce SWD pressure.
Non-crop hosts from areas surrounding your fruiting crop may be an issue—Potential perimeter, wildland and backyard uncultivated plants used by SWD may include berries from: dogwood, elderberry, Himalayan blackberry, laurel, sweet box (Sarcococca spp.), flowering cherry, honeysuckle, and dozens of other fruiting species. See OSU Extension publication EM9096: Noncrop Host Plants of Spotted Wing Drosophila in North America. (https://catalog.extension.oregonstate.edu/em9096). Some of these fruits may not be affected by SWD under certain environmental conditions or because of specific management practices being used.
Canopy and irrigation management— SWD prefer shady and humid habitats. Maintain an open and aerated plant canopy that is less attractive to SWD adults and minimize leaky irrigation lines and overhead irrigation. Use drip irrigation, as it increases temperature and reduces water availability to adult flies. Drip irrigation also results in increased host feeding of pupal parasitoids, ultimately resulting in higher mortality of SWD.
Cooling fruit—Chill fruit (less than 34°F) immediately after harvest for extended time periods (greater than 4 to 8 days) but retaining fruit quality to slow or kill eggs and young larvae.
Management—biological control
Research is underway to determine the specific predators and parasitoids (wasps) that attack SWD larvae and pupae. Field observations suggest that ants, spiders, predaceous bugs (e.g., minute pirate bugs, big-eyed bugs), yellow jackets, lacewing larvae, and parasitoid wasps may be important biological control agents.
Management—chemical control
Chemical controls should be coupled with monitoring efforts. Rotate chemical families to avoid resistance and follow the label for each crop. See specific fruit for recommended chemicals.
Pesticide families that help control SWD include: spinosyns, pyrethroids, carbamates, and organophosphates. These chemicals kill SWD adults, and some may have an effect on larvae that are developing within and are protected by the fruit. Follow the label for appropriate rates and risks. Do not apply when bees and other pollinators are present, such as when plants are flowering or when pollinators are active.
For further information:
Mermer, S., L. Brewer, D. Dalton, R. Nieri, K. Park, F. Pfab, M. V. Rossi-Stacconi, and V. Walton. 2019. Improved Chemical Control Strategies for Spotted-wing Drosophila. Oregon State University Extension Service EM 9265.
Mermer, S., G. A. Hoheisel, H. Y. Bahlol, L. Khot, D. Rendon, L. Brewer, D. Dalton, R. Nieri, K. Park, F. Pfab, M. V. Rossi-Stacconi, and V. Walton. 2019. Optimizing Chemical Control of Spotted-wing Drosophila. Oregon State University Extension Service EM 9266.
Rendon, D., S. Mermer, L. Brewer, D. Dalton, C. B. D. Silva, J. Lee, R. Nieri, K. Park, F. Pfab, G. Tait, N. Wiman, and V. Walton. 2019. Cultural Control Strategies to Manage Spotted-wing Drosophila. Oregon State University Extension Service EM 9262.
Rossi-Stacconi, M. V., L. Brewer, D. Dalton, J. Lee, R. Nieri, K. Park, F. Pfab, G. Tait, and V. Walton. 2019. Host Range and Characteristics Affecting Fruit Susceptibility to Spotted-wing Drosophila. Oregon State University Extension Service EM 9263.
Rossi-Stacconi, M. V., L. Brewer, B. Miller, D. Dalton, J. Lee, K. Park, F. Pfab, V. Walton, and C. B. D. Silva. 2019. Biocontrol of Spotted-wing Drosophila. Oregon State University Extension Service EM 9229.
Silva, C. B. D., B. E. Price, D. Dalton, D. Rendon, K. Park, L. Brewer, V. Walton, and M. V. Rossi-Stacconi. 2019. Potential Impacts of Irrigation and Biocontrol on Spotted-wing Drosophila Populations. Oregon State University Extension Service EM 9268.
Tait, G., D. Rendon, L. Brewer, D. Dalton, J. Lee, R. Nieri, K. Park, F. Pfab, M. V. Rossi-Stacconi, and V. Walton. 2019. Noncrop Host Plants Used By Spotted-wing Drosophila. 3.
Tait, G., M. V. Rossi-Stacconi, B. Miller, D. Dalton, J. Lee, K. Park, V. Walton, T. Peerbolt, and L. Brewer. n.d. Monitoring Techniques for Spotted-wing Drosophila. Oregon State University Extension Service EM 9267.
Walton, V., L. Brewer, D. Dalton, S. Tochen, R. Nieri, K. Park, F. Pfab, D. Rendon, G. Tait, N. Wiman, and M. V. Rossi. 2019. How Seasons Affect Population Structure, Behavior and Risk on Spotted-wing Drosophila. Oregon State University Extension Service EM 9261.